DNAwiz
What is DNAwiz?
Our recently developed DNAwiz is part of our GIMS platform and improves processes of any NGS-based diagnostic: germline and somatic. This includes genetic diagnostics of rare hereditary diseases, cancer predispositions and sequencing analyses of tumor materials aswell. It harbours structured step by step decision-making and documentation-workflows for variant classification, clinical interpretation and recommendation.
DNAwiz enables molecular and clinical teams to work together in a structured, digital genetic ecosystem - laying the basis for better medical decisions. Aggregated knowledge and findings become transparently trusted and disseminated: the fair sharing of variant information, genetic knowledge and clinical observations is part of a continuous truth-finding process.
How it works

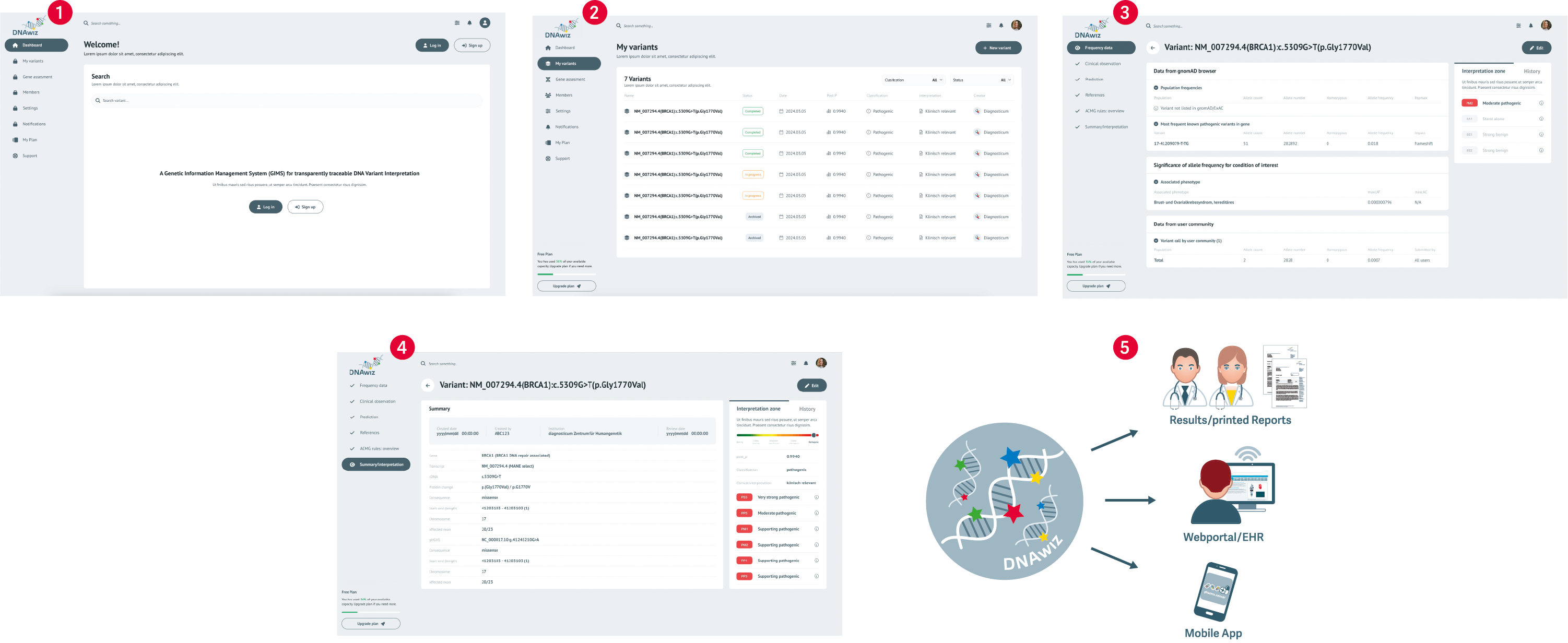
1. variant list (vcf) is uploaded with standard interfaces or manually
2. detected variants are listed and automatically connected with metadata from public sources
a) an already available and trustfully classified variant is automatically processed for generation of a report in clinical
grade
b) step by step guidance through the variant assessment and interpretation process for processing formerly not classified
variants
>decision and argumentation processes can be documented and versionised
c) if possible, the user can also draw variant information and existing assessments from the sharing network
3. completed assessments can be reviewed and approved using customizable workflows
a) once assessments have reached a level of maturity, the costumer can choose to share the knowledge within the network
4. interpretations, diagnosis and recommendations are compiled in a clinical report
a) available in multiple formats using content from the database amended with the user's comments where applicable
5. results can be...
a) provided to physicians and patients as printed documents
b) incorporated into EHR systems
c) distributed using customizable digital channels
Key Benefits
PRODUCTIVITY AND TIME SAVINGS
- stepwise guidance through variant classification and interpretation process
- automated connection of variants and metadata from public sources and the production of diagnostic reports
Structured documentation
- clear-cut workflows for review and approval processes
- enables standardization and constant quality of diagnostic output
- structured compilation of own knowledge
TRANSPARENCY AND CREDIBILITY
- reproducible decision processes (ideal for QA and audits)
- sharing of content and knowledge within a network of laboratories, scientific institutions and expert groups
Contact block
PLEASE NOTE: By filling out the contact form and activating the send button you consent to the processing of the stated data according to art. 6, para. 1a EU-GDPR for the purpose of processing your inquiry. More information can be found in our privacy policy.

